Re-orchestrating neural networks to counter schizophrenia
18 December 2017
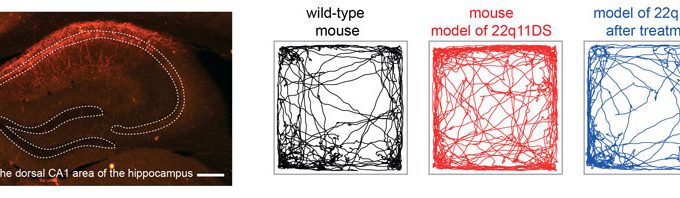
A three-level study of the synaptic structure, neural network and behavior is particularly helpful for understanding neuropsychiatric diseases, including schizophrenia and patients with 22q11 microdeletion.
The structural properties of neurons at the synaptic scale influence their effective and functional connectivity. Knowing how this link affects the dynamics of neural networks and how it is reflected in behavior is the hobby horse of Thomas Marissal and the group led by Alan Carleton at the University of Geneva. The Geneva researchers are tackling the issue by working on an animal model: the LgDel mouse line that carries 22q11 microdeletion. These mice have known defects in their synaptic structure, and Marissal soon observed in an in vitro experimental model (an organotypic hippocampal culture) that the network activity was decorrelated. “The neurons,” explains Marissal, “tend to be out of sync in 22q11 mice. They don’t ‘sing along’ together although individually they function normally”.
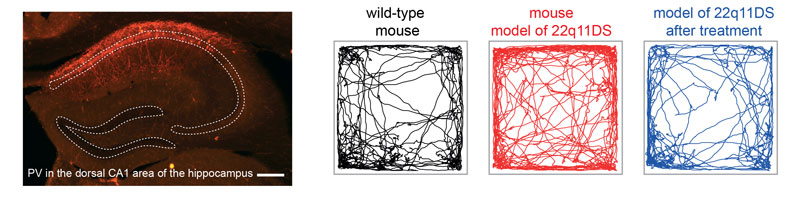
Figure left: Virally-labelled Parvalbumin interneurons (red) in the CA1 area of the hippocampus from mouse model of 22q11DS.
Figures right: Locomotor tracks of a wild-type mouse (black), a mouse model of 22q11DS untreated (red) or after treatment (blue). Note the hyper-locomotor behavior of the mouse model of 22q11DS, which is reduced after treatment.
A cellular conductor
To understand the basis of this observation, Marissal immediately thought that there must be a conductor directing the global neural activity. Parvalbumin (PV) neurons, which represent 1% of the neuronal population of the hippocampus, are inhibitory neurons known to govern the activity of the excitatory neurons. They were quickly identified by the neuroscientist as ideal candidates: “They are the switchboard operators for the neural network,” says Marissal.
On studying these neurons, Marissal discovered alterations in their physiological properties. Specifically, the PV neurons of the 22q11 mice are hyper excitable thus generating fewer action potentials. Since the exogenous modulation of neuronal excitability is technically easy, whether pharmacologically or genetically, Marissal and his colleagues were able to play with the excitability of PV neurons. They succeeded in reversing the hyperactivity and demonstrating that it was possible to reverse the synchronization problem of the excitatory neurons. They then established the causal link between neuronal desynchronization and behavioral disorders by managing to drastically reduce the behavioral symptoms associated with 22q11 deletion until a return to normal. Marissal was a very happy man, declaring: “Our results are fantastic! The 22q11 mice are indivisible from the control mice after treatment”.
The strength of the Synapsy’s network
These behavioral disorders concern learning, anxiety and somatosensory integration problems which can be directly transposed to humans. In addition, some of the behavioral tests carried out on mice may be applied to humans without being modified, suggesting that there is significant translational potential. Although Alan Carleton’s group is keeping silent about the name of the pharmacological treatment given to the mice, it is a known molecule that acts specifically on PV neurons. It works in adults and not during a developmental phase, as is the case for treatments assessed by Pico Caroni and Stephan Eliez on sub-populations of PV neurons. There is a clear correlation between the two research groups, with shared treatment pathways and exciting research synergies. The fact that the molecule is already known implies that the possibilities of technology transfer and filing a patent are, unfortunately, very limited. On the other hand, a collaboration with the group led by Eliez and the 22q11ds clinical cohort could lead to future – and already promising – treatment trials on humans.
Author : Yann Bernardinelli
